LIDE specializes in preclinical oncology studies, offering traditional R&D in-vitro and in-vivo services but recommend a different R&D roadmap for evaluating potential oncology treatments:
- Using conditionally reprogrammed (CR) cells instead of genetically engineered cell lines for in-vitro studies. Many of our cell lines have matching PDX models.
- Bridging in-vitro to in-vivo studies by validating your compounds using MiniPDX, an in-vivo version of an organoid assay. Drug sensitivity results in 7 days.
- Preparing your IND filing with the gold standard PDX study.
- Leveraging MiniPDX and fresh patient samples in a biomarker identification study, to inform patient stratification and recruitment strategy for clinical trials
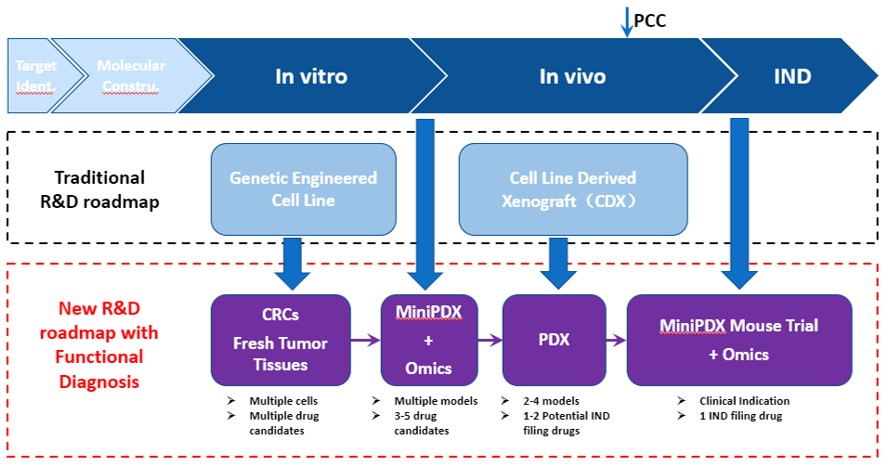
Fig. New, optimized R&D roadmap using LIDE Functional Diagnosis platform and conditional reprogramed (CR) cells
Oncology studies done with LIDE also benefit from our Translational Medicine platform that's provided LIDE with models with drug resistance, high profile expression targets or of rare cancer types.