huPBMC reconstitution is a good model for evaluating therapeutic antibody, especially the BsAb with one arm of anti-CD3, that mediates immune cells. However, limited window of dosing regimen triggered by GvHD after 30-40 days of reconstitution, and insufficient tumor-infiltrated immune cells naturally from reconstituted immune cells in mice circulation hinders the widely application of the model in immunotherapy in development of multiple immune checkpoint inhibitors (e.g.> TIGIT, PVRIG, CD73, etc.).
To overcome this, LIDE has developed huPBMC-cancer cells co-inoculated models instead. In this platform, huPBMC will be firstly co-cultured with cancer cells. Then the mixture of the activated huPBMC and the cancer cells will be co-inoculated into mice to form a huPBMC well-infiltrated tumor tissue for immunotherapy.

Flowchart of huPBMC-cancer cells co-inoculated CDX/PDX model
LIDE has developed several models based on the co-inoculated platform, and has supported a great number of client projects with multiple immune-oncology targets.
| Humanized CDX/PDX model available in LIDE |
| A375 | MDA-MB-231 |
| SHP77 | Daudi |
| Molp8 | SK-MEL-5 |
| A549 | Raji |
| ES-2 | HepG2 |
| MKN-45 | U87 |
| Lovo | HEK293-Claudin 18.2 |
| Huh7 | NCI-N87 |
| PC-3 | PDXs in LIDE |
| To be continued... |
| I/O target available in LIDE |
| PD-1/PD-L1 | TIGIT |
| GITR | PVRIG |
| TIM-3 | CSF1R |
| CD29 | CD47/SIRPa |
| CD38 | CD39 |
| CD40 | B7H3 |
| CD73 | LAG3 |
| IL2 | IL-15 |
| TGFβ/PD-L1 | CD3/Claudin 18.2 |
| CD3/GPC3 | CD3/DLL3 |
| PD-L1/CD47 | CD3/EpCAM |
| To be continued... |
A375 and huPBMC co-inoculating I/O model is an ideal platform for evaluation of many kinds of monoclonal antibodies.
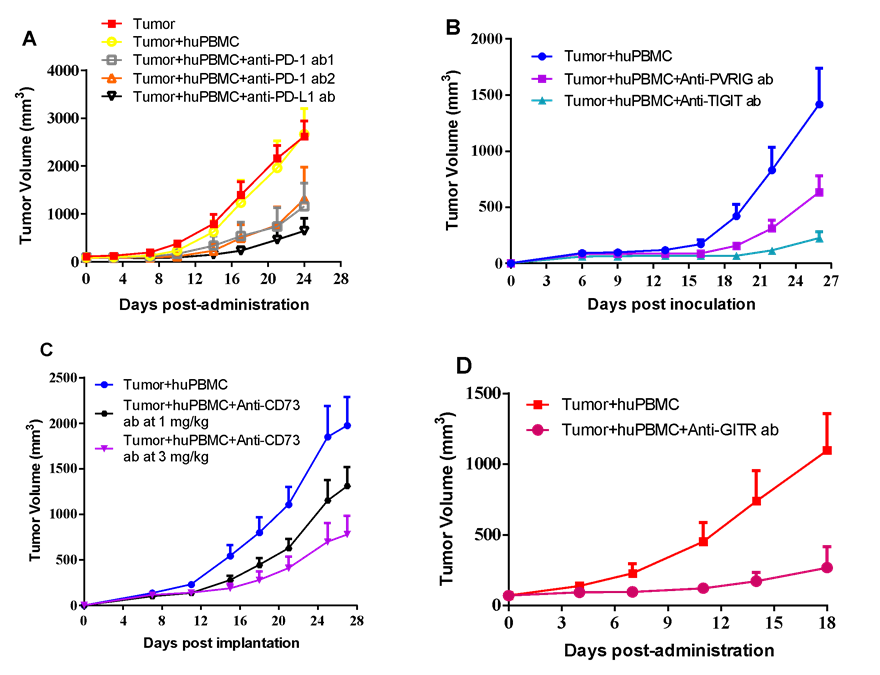
huPBMC from indicated donors were co-cultured with A375 human melanoma cancer cells in vitro under specific condition. The activated huPBMC were then harvested and mixed with A375 cancer cells in certain E:T ratio, and co-inoculated into the right flank in NCG mice. Mice were randomized immediately after tumor co-inoculation or when average tumor volume grown up to 50-100 mm3, with dosing initiated accordingly, while tumor volume and body weight were measured twice a week. (A) Anti-PD-1 antibody from two vendors and anti-PD-L1 antibody were dosed at 10 mg/kg via i.p. bi-weekly for a total of 6 injections. (B) Anti-PVRIG antibody at 16.1 mg/kg or anti-TIGIT antibody at 30 mg/kg were dosed via i.p. every other day for a total of 12 injections. (C) Anti-CD73 antibody at 2 dose levels were dosed via i.p. every other day for a total of 11 injections. (D) Anti-GITR antibody at 1 mg/kg was dosed via i.p. weekly for a total of 3 injections.
Bi-specific antibodies can also be evaluated in co-inoculating platforms using different cancer cell lines that express indicated bio-markers.
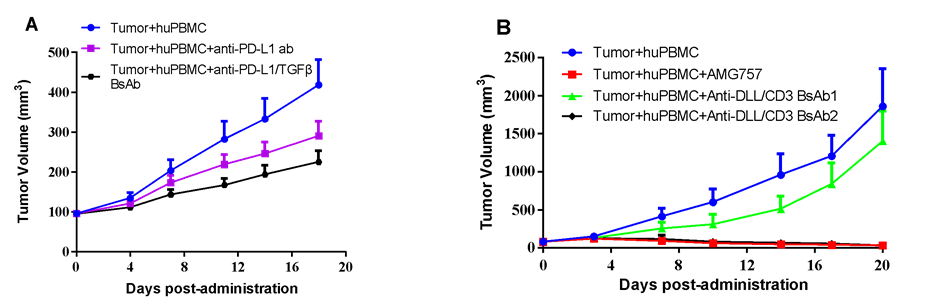
huPBMC from indicated donors were co-cultured with MDA-MB-231 human breast cancer (A) or SHP-77 human SCLC (B) cells in vitro under specific condition, and co-inoculated accordingly. Mice were randomized when average tumor volume grown up to 100 mm3, with dosing initiated accordingly, while tumor volume and body weight were measured twice a week. (A) Anti-PD-L1/TGFβ antibody at 6 mg/kg or its isotype anti-PD-L1 antibody at 5 mg/kg were dosed via i.p. every other day for a total of 9 injections. (B) AMG757 at 1.6 mg/kg or two anti-DLL/CD3 BsAb at 0.5 mg/kg, respectively, were given via i.p. bi-weekly for a total of 6 injections.
LIDE established 1400+ PDX can also be utilized in huPBMC co-inoculating models for antibody evaluation.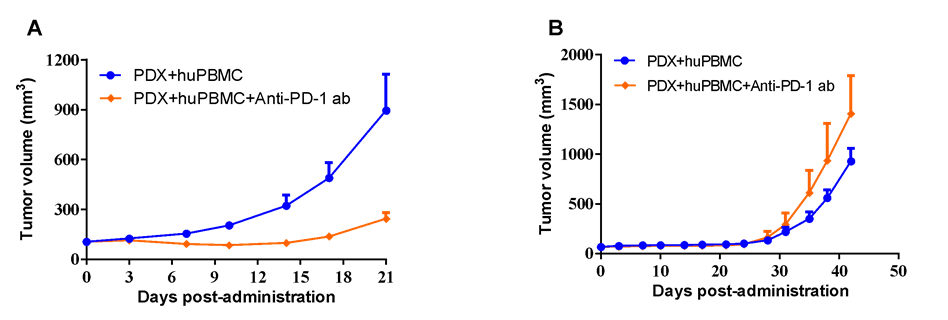
#LD1-0006-215648 human NSCLC PDX model with high-expression of PD-L1 (A) or #LD1-0025-200613 human NSCLC PDX model with acquired-resistance to anti-PD-L1 antibody in clinical practice (B) were co-inoculated with activated huPBMC, while anti-PD-1 antibody at 20 mg/kg via i.p. every other day for a total of 10 injections were applied for anti-tumor validation.