LIDE has established 1900+ high quality PDX models in almost 50 cancer types – the second largest PDX bio bank in the world.
| Cancer | No. | Cancer | No. | Cancer | No. |
| Acute lymphoblastic leukemia | 12 | Extramedullary tumor | 1 | Multiple Myeloma | 3 |
| Acute myelocytic leukemia | 16 | Fallopian tube carcinoma | 2 | Neuroendocrine tumor | 6 |
| Adenoid cystic carcinoma (ACC) | 5 | Gallbladder carcinoma | 36 | Osteosarcoma | 58 |
| Bladder cancer | 5 | Gastric cancer | 202 | Ovarian cancer | 203 |
| Brain cancer | 69 | Gastrointestinal stromal tumor | 6 | Paget's Disease | 1 |
| Breast cancer | 45 | Hepatoblastoma | 42 | Pancreatic cancer | 305 |
| Cervical cancer | 55 | Hepatocellular | 103 | Penile cancer | 2 |
| Cholangiocarcinoma | 82 | Head and neck cancer | 7 | Periampullary carcinoma | 2 |
| Chordoma | 2 | Lung cancer | 161 | Prostate cancer | 7 |
| Chronic Lymphocytic Leukemia | 2 | Lymphoma | 16 | Rectal Cancer | 81 |
| Colon cancer | 116 | Malignant mesothelioma | 2 | Renal carcinoma | 9 |
| Duodenal carcinoma | 15 | Mediastinal endoblastoid sinus tumor | 1 | Sarcoma | 38 |
| Endometrial cancer | 17 | Melanoma | 4 | Spleen cancer | 1 |
| Esophageal cancer | 42 | Mucinous carcinoma | 5 | Urethral carcinoma | 4 |
Table: Summary of available PDX Models
Leverage our patient derived xenograft models and partner with LIDE on your next oncology research project:
- In vitro TCA compounds screening
- In vivo efficacy study
- Potential indication screening via MiniPDX assay
- Immunotherapy evaluation on huPBMC reconstituted models
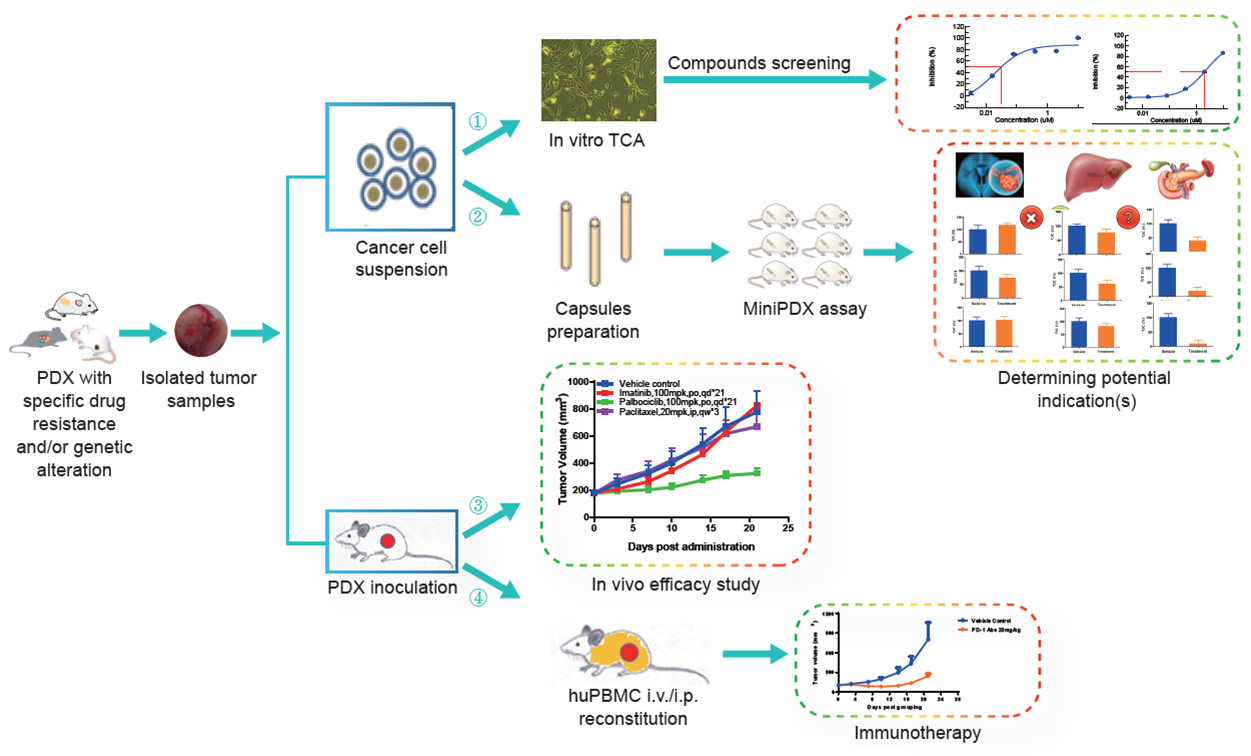
Fig. Schematic of oncology R&D projects available through PDX models
Why LIDE Models:
- Many models of rare indications, drug resistant or relapsed/recurrent cancer
Because we have a MiniPDX® testing program with hospitals all over China, LIDE has been able to amass a large collection of rare 3R (resistant, relapsed, recurrent) models for testing of potential 2nd or 3rd line treatments. - Highly-specific molecular targets and hard to find genetic alterations
LIDE has validated 200+ special drug resistant and/or genetic altered PDX models, including EGFR single/double/triple mutations in lung cancer, triple negative breast cancer, and models with KRAS mutation, HER2 amplification, or ALK/ROS1/NTRK fusion, etc.
Table: LIDE resistant and genetically modified PDX modelsCancer Type Resistant to Specific Genetic Alteration NSCLC Eriotinib
Osimertinib
Crizotinib
Brigatinib
anti PD-L1 abEGFR: exon19del/T790M/L858R/exon20ins/ C797S
ALK: EML4-ALK/L1196M
cMET: ampli./exon14ski/CD47-MET
RET: KIF5B-RET
ROS1: CD74-ROS1/G2032R
KRAS: G12C
PTEN: Y68X
P13K: E726KBreast Cancer CDK4/6i TNBC/ER+ Multiple Myeloma Bortezemonib CD47+/CD38+ Cholangiocarcinoma Paclitaxel KRAS: G12C
FGFR: BICC1-FGFR2Colorectal Cancer Avastin KRAS: G12C
BRAF: V600EHematological Malignancy Rituximab
Imatinib/ Gastric Cancer Herceptin HER2: ampli
KRAS: G12CBrain Cancer / EGFR: VII
cMET: PTPRZ1-METMelanoma anti PD-1 ab BRAF: V600E Ovarian Cancer Platinum
PARPi/
- Highest testing standards
Pathological analysis will be done using H&E staining from FFPE reserved during each passage of individual PDX models by a certified pathologist under the U.S. CAP standard. WES and RNAseq will be done in most of the established models according to the highest international standards of PDX Consortium.
Download a LIDE PDX FactSheet Download a LIDE PDX and Cell Line List