LIDE does not offer clinical testing services but offers several preclinical services that will optimize your clinical trials:
- Indication screens using genetically modified or drug resistant models
- Biomarker identification to guide patient stratification and recruitment strategy
Traditional oncology clinical trials are either basket trials or umbrella trials.
- Basket trials test multiple tumors with similar molecular abnormalities, to identify the best indications for treatment.
- Umbrella trials test a single tumor type but different molecular biomarkers, to identify best responders.
LIDE’s Functional Diagnosis platform offers complementary insights as basket and umbrella trials, prior to the clinical trial phase:
- MiniPDX Mouse Trial – fast, cost-effective drug screens with similar predictive power as traditional PDX
- OMICS analysis – identifying biomarkers through advanced bioinformatics analysis at all levels of transcription
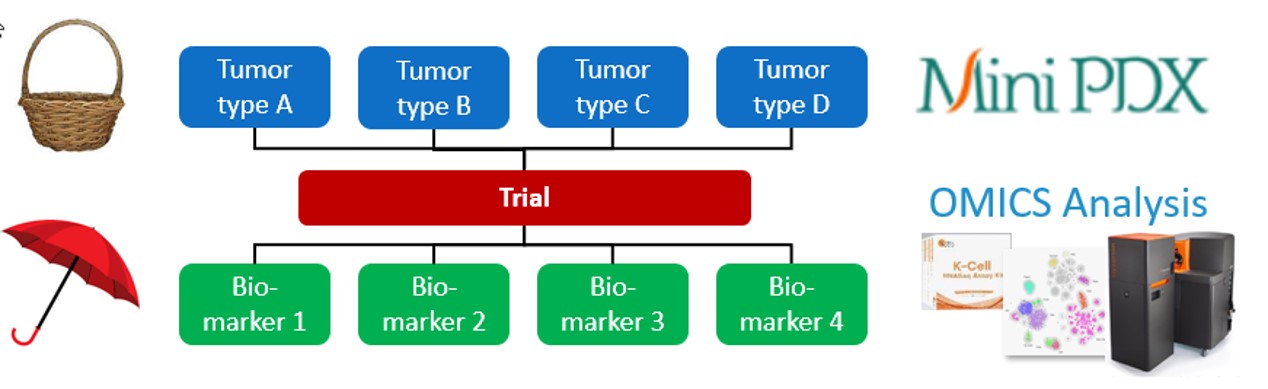
Fig. LIDE Functional Diagnosis platform can offer complementary insights to basket and umbrella trials but in preclinical stage.