CDX or PDX models can be utilized for immunotherapeutic evaluation when tumor-bearing mice were reconstituted with huPBMC via i.p./i.v. injection.
 Flowchart of huPBMC reconstituted CDX/PDX model
Flowchart of huPBMC reconstituted CDX/PDX model
Consistency of interaction between immune cells and cancer cells is of essential to success of immune-oncology (I/O) projects when using huPBMC reconstituted or co-inoculated platforms. LIDE performs initial investment for antibody validation in various batches of huPBMC and diverse mice strain, all of which enables LIDE to provide high-quality and stable platforms for client to complete their projects by utilizing the same batch of validated huPBMC.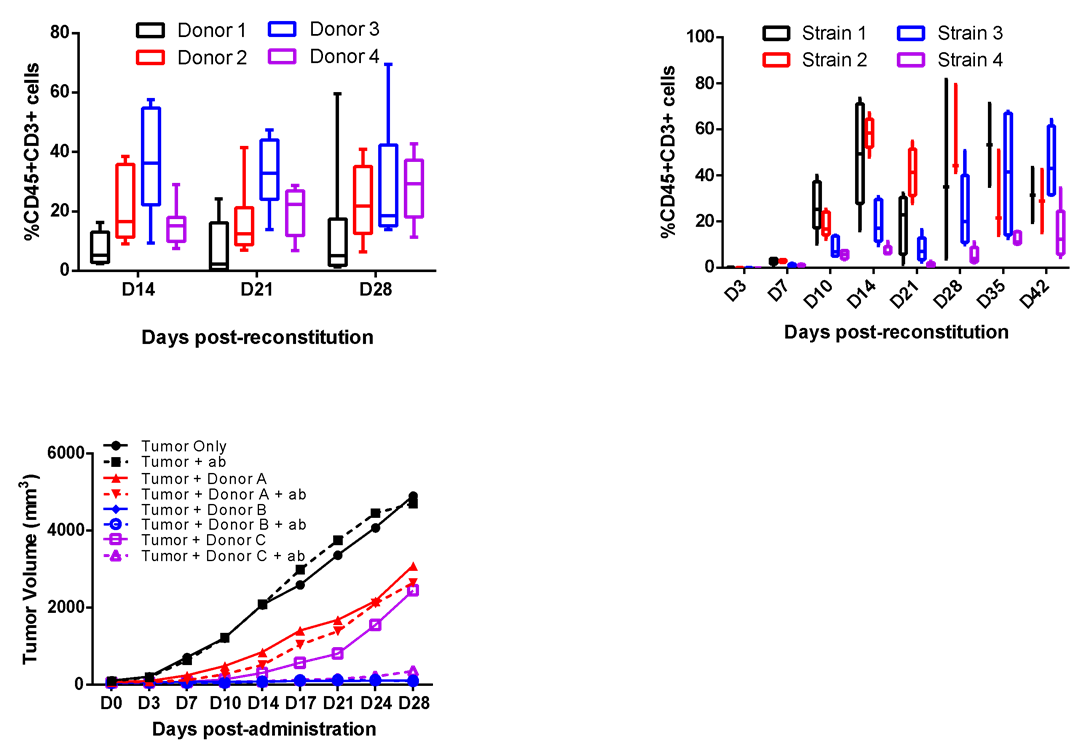 LIDE has ordered over 10 batches of commercialized huPBMC, and performed validation in different mice strain (Left and middle). Validated strain has been reconstituted with varied donor’s PBMC and treated with validation antibody to confirm the best donor for immunotherapy (right).
LIDE has ordered over 10 batches of commercialized huPBMC, and performed validation in different mice strain (Left and middle). Validated strain has been reconstituted with varied donor’s PBMC and treated with validation antibody to confirm the best donor for immunotherapy (right).
Using validated platforms, LIDE provide in vivo efficacy assay for client to evaluate immunotherapy about investigated antibodies.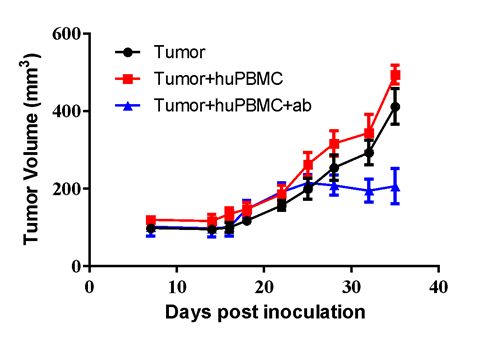
#LD1-0010-200614 human cervical PDX model was inoculated into the right flank in NOG mice. Mice were reconstituted with huPBMC via i.p. 7 days after tumor inoculation, while animals were randomized when average tumor volume grown up to ~150 mm3, with dosing initiated accordingly. An investigated BsAb was dosed at 0.125 mg/kg through i.p. once a day for a total of 18 injections. Tumor volume and body weight were measured twice a week.