The primary benefit of CR (conditionally reprogrammed) cells is its intratumor and intertumor heterogeneity, even after amplification. This means CR cells can be used instead of genetic engineered cells or commercialized cancer cell lines in traditional patient-derived cancer models like PDX.
LIDE validated this separately to ensure established CR cells demonstrate heterogeneity like its parental PDX tumors. Below is an example of EFGF mutant PDX models and CR cell lines, showing model drug sensitivities are similar.
| Model ID | EGFR status |
| LD1-0025-200636 | WT |
| LD1-0006-215676 | L858R/T790M |
| LD1-0025-200717 | 19del/T790M/C797S |
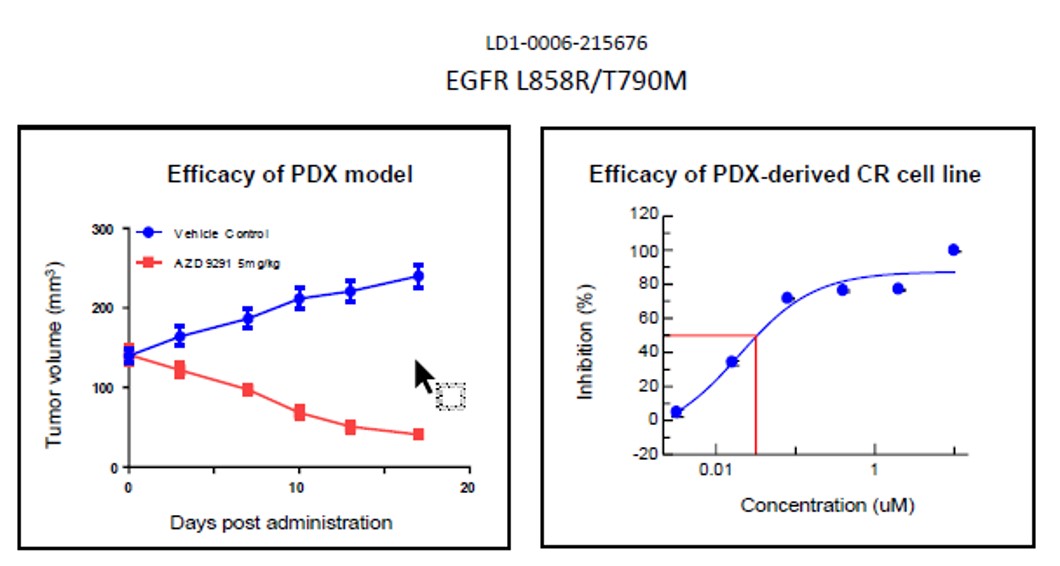
Fig. Efficacy results of LD1-0006-215676. IC50 of CR cell line was .03, indicating drug potency
and matching efficacy result of PDX model
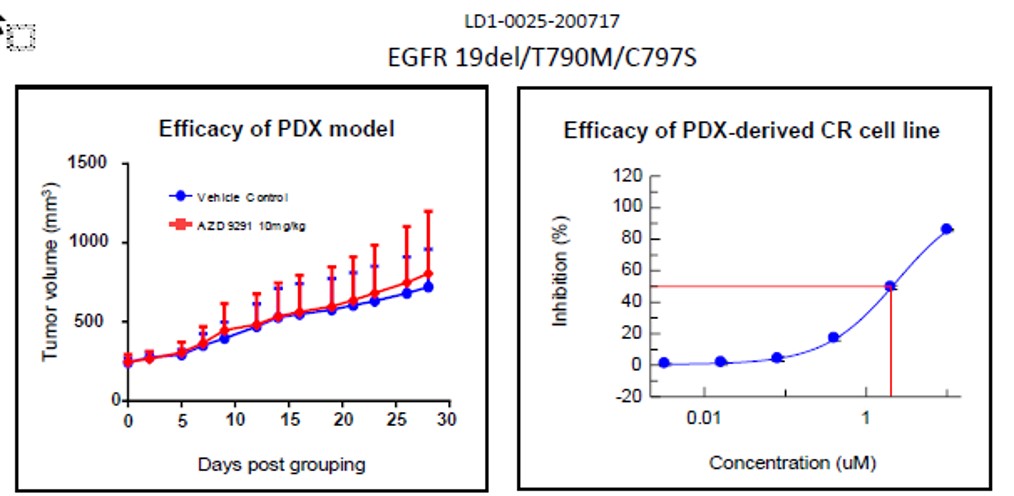
Fig. Efficacy results of LD1-0025-200717. IC50 of CR cell line was 2.01, indicating drug
insensitivity, as expected and matching result of PDX model
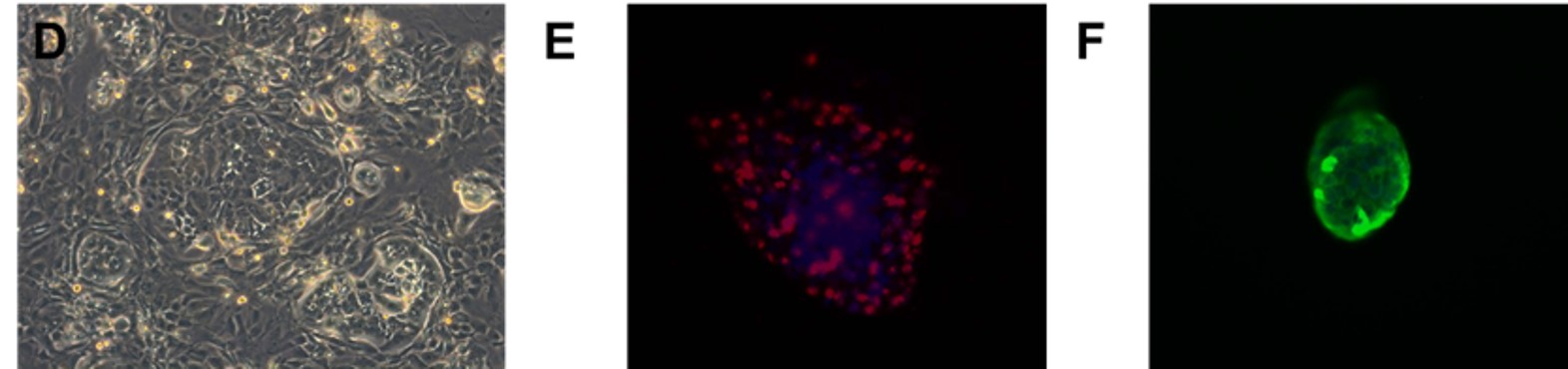
Fig. Morphology of EGFR 19del/T790M /C797S PDX derived cell line (D); Ki67 (E) and Pan-CK (F) staining of 19del/T790M /C797S PDX matching cell derived tumor sphere.