Patient Stratification with Companion Diagnosis and Omics Analysis:
Even if drug companies successful match promising drug candidates with the correct indications, clearing FDA approval requires a smart clinical trial strategy, especially regarding patient selection and recruitment.
LIDE offers robust “omics” studies from our bioinformatics team that can analyze positive and negative responders at all levels of transcription, to identify biomarkers that can be used in patient stratification.
- Standard genomic level DNA sequencing and analysis
- Transcriptome level analysis with FGI , a differentiated analysis platform that groups expression data into functional units and a clickable UI for further exploration
- Protein-level analysis using state-of-the-art mass-cytometry
These studies are routinely paired with MiniPDX indication screens because we can leverage the same tissue samples but extend the research and analysis possible. Our OncoVee K-cell Kit allows DNA/RNA extraction from small biopsy samples, requiring only 1000’s of cells vs. the 10-100’s of thousands usually required.
Learn more about our K-cell 1000 cell kits
Case Study:
Pharma Company X had developed candidate “cpd” that demonstrated good efficacy against Cancer Type A in CDX models. Literature research also showed that the molecule could be effective against three other cancer types. LIDE designed a Functional Diagnosis study, leveraging MiniPDX to isolate specific indications and FGI to identify any associated companion diagnosis.
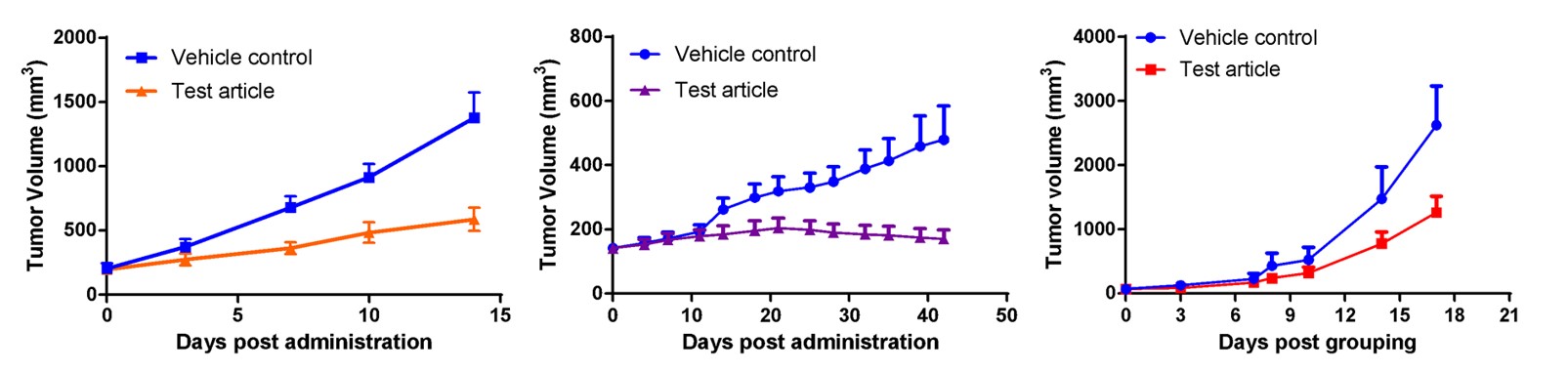
Fig. CDX results for candidate “cpd” showing efficacy for Cancer Type A
In initial round of MiniPDX® testing, 31 samples across the four cancer types were treated with cpd. Overall ORR (overall response rate) in round one was 16.1%; only five cases were positive responses. Notably none were in Cancer Type A, the original candidate indication as identified by CDX. Cancer Type A was quickly abandoned while 10 additional samples of Cancer Type B were added for further testing.
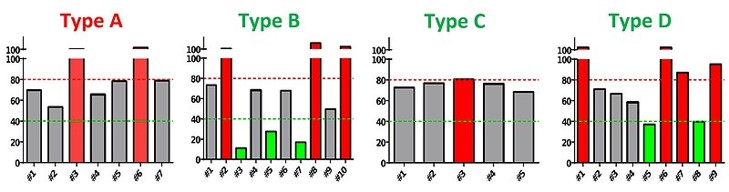
Fig. Initial MiniPDX® results for 31 samples across four cancer types; 16.1% ORR with five positive (green) responses.
In round two, testing was done only on Cancer Type B. Out of 20 cases, ORR was 35%, or 7 out of 20 positive responses. Although this was a MiniPDX® model, 35% ORR in PDX studies would’ve cleared benchmarks required for clinical trial approval.
LIDE then took both the negative and positive responders, leveraged our OncoVee™ K-cell technology and Functional Genomic Imaging omics analysis, to reveal that positive responders were differentiated with downstream mutation E1/E2. This was a clear companion diagnostic that can be used to select patients and ensure success in clinical trials.
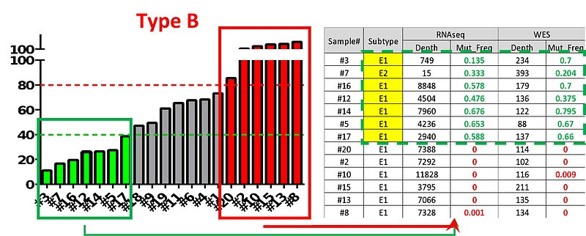
Fig. Further testing of Cancer Type B against cpd, combined with genomic analysis of positive and negative responders to reveal companion diagnostic.
By selecting patients with this biomarker in clinical trials, theorized success rate can be close to 100%. Even if not that high, odds are stacked in favor of success by pairing the right drug with the right patients.