LIDE has established 1700+ PDX models. WES and RNAseq profiling of established PDX models has identified many mouse models that harbor specific gene alterations. See below for a partial list but contact us for the most updated.
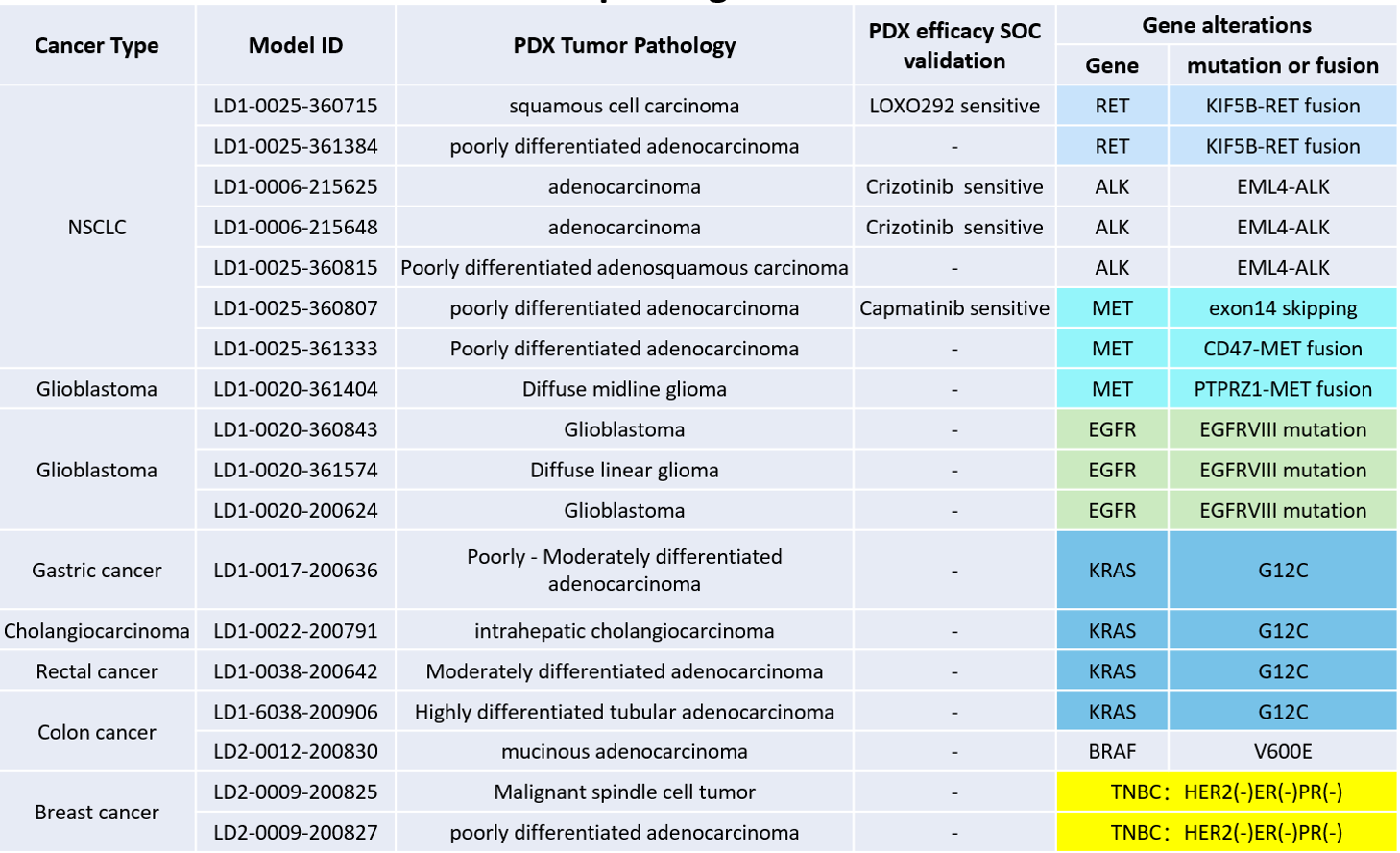
LIDE has established 1700+ PDX models. WES and RNAseq profiling of established PDX models has identified many mouse models that harbor specific gene alterations. See below for a partial list but contact us for the most updated.
